
#Iso 17025 2017 mandatory documents iso#



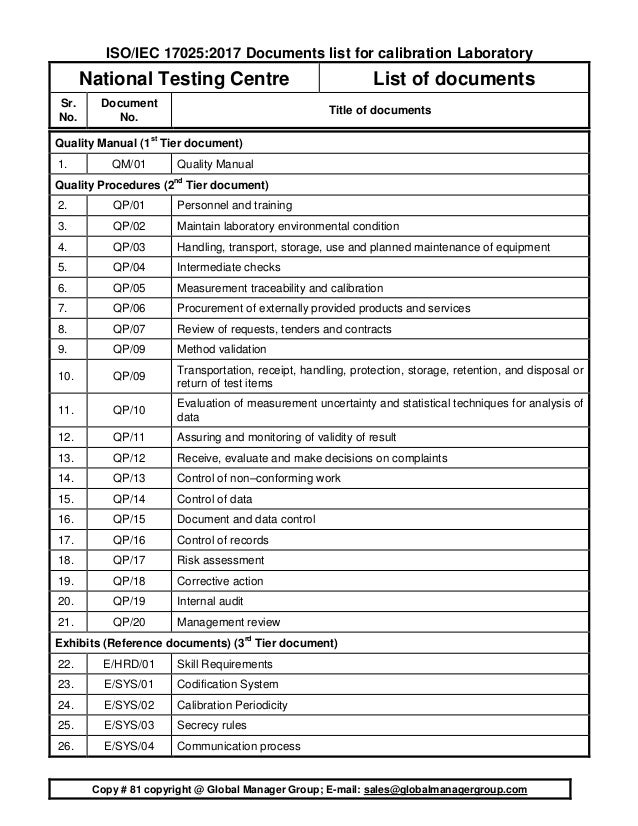
Standard Operating Procedures (02 sops): It includes operating procedures for good work practices.Exhibits (8 exhibits): The 8 exhibits including an exhibit for calibration periodicity of instruments.It covering the mandatory requirements of documented procedures. ISO 17025 Procedures (20 procedures): It incluprocedures to implement the system in the test laboratory and comply with accreditation standard requirements.
#Iso 17025 2017 mandatory documents manual#
Each ISO 17025 manual chapter is explaining macro-level management strategy and commitment and how the laboratory system is implemented in plain English. ISO 17025 Manual (with 8 chapters): A sample laboratory quality system - ISO 17025 2017 manual with the quality policy.The contents of the environmental / chemical testing laboratory document kit include more than 100 document files as listed below: The lists of various types of documents are listed below.Our documents are editable and many organizations and ISO 17025 consultants are using our documents. The ISO 15189:2012 documents, requirement for medical laboratories quality management system can accelerate documentation process for ISO 15189 Accreditation. The lists of various types of documents are listed below.ĭ135 : ISO 15189:2012 Documentation Kit for Medical Laboratory - 450 USD What to include in ISO 15189:2012 Documents The ISO 17065:2012 documents, requirements for bodies certifying products, processes and services can accelerate documentation process for ISO 17065 Accreditation. The lists of various types of documents are listed below.ĭ134 : ISO 17065 Documentation Kit for Product Certification Bodies - 810 USD What to include in ISO 17065:2012 Documents The ISO 17043:2010 documents for ISO 17043 Accreditation can be easily implemented using 4 tier documentation structure. The lists of various types of documents are listed below.ĭ130 : ISO/IEC 17043:2010 Documentation Kit for Proficiency Testing Provider - 999 USD What to include in ISO 17043:2010 Documents ISO 17034:2016 documents required for accreditation of reference material producers based on ISO 17034:2016 Standard can be easily implemented using 4 tier documentation structure. The lists of various types of documents are listed below.ĭ132 : ISO 17034:2016 Documentation Kit for Reference Material Producer - 899 USD What to include in ISO 17034:2016 Documents The ISO 17024:2012 documents need to make good system and establish complete documentation for ISO 17024 Certification system establishment. ISO 17021:2015 documents which conforming to the requirements of conformity assessment of Certifying Bodies as per ISO 17021:2015 are listed below.ĭ128 : ISO 17024 Documentation Kit for Personnel Certification - 999 USD What to include in ISO 17024:2012 Documents Exhibits and Standard Operating Proceduresĭ121 : ISO 17021:2015 Documentation Kit for Certifying Body - 720 USD What to include in ISO 17021:2015 Documents.Conformity assessment mandatory procedures.Sample assessment manual for inspection agency.The following list of ISO 17020:2012 documents can be easily implemented using 4 tier documentation structure for ISO 17020 Accreditation. Work Instructions / Standard operating proceduresĭ118 : ISO 17020:2012 Documentation Kit for Inspection Agency - 450 USD What to include in ISO/IEC 17020 Documents.The lists of various types of documents are listed below. The ISO 17025:2017 documents for ISO/IEC 17025 certification can be easily implemented using 4 tier documentation structure. D119 : ISO 17025:2017 Documentation Kit for Testing Laboratory - 699 USD What to include in ISO/IEC 17025 Documents


 0 kommentar(er)
0 kommentar(er)
